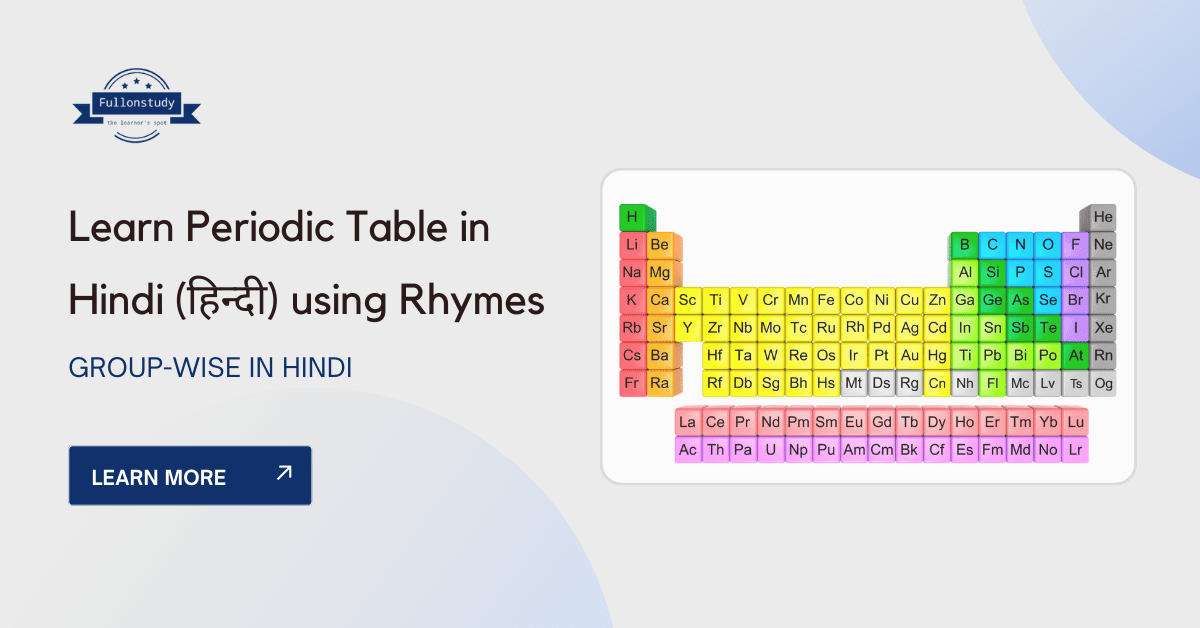
How to learn Periodic Table in Hindi – [Short Tricks]
How to learn Periodic Table in Hindi? The periodic table is the ultimate guide to the world around us. It’s a blessing and a curse. A blessing because the elements […]
Here is the list of all the posts with category Chemistry from Fullonstudy. Below is the list of all the posts under this category.

How to learn Periodic Table in Hindi? The periodic table is the ultimate guide to the world around us. It’s a blessing and a curse. A blessing because the elements […]